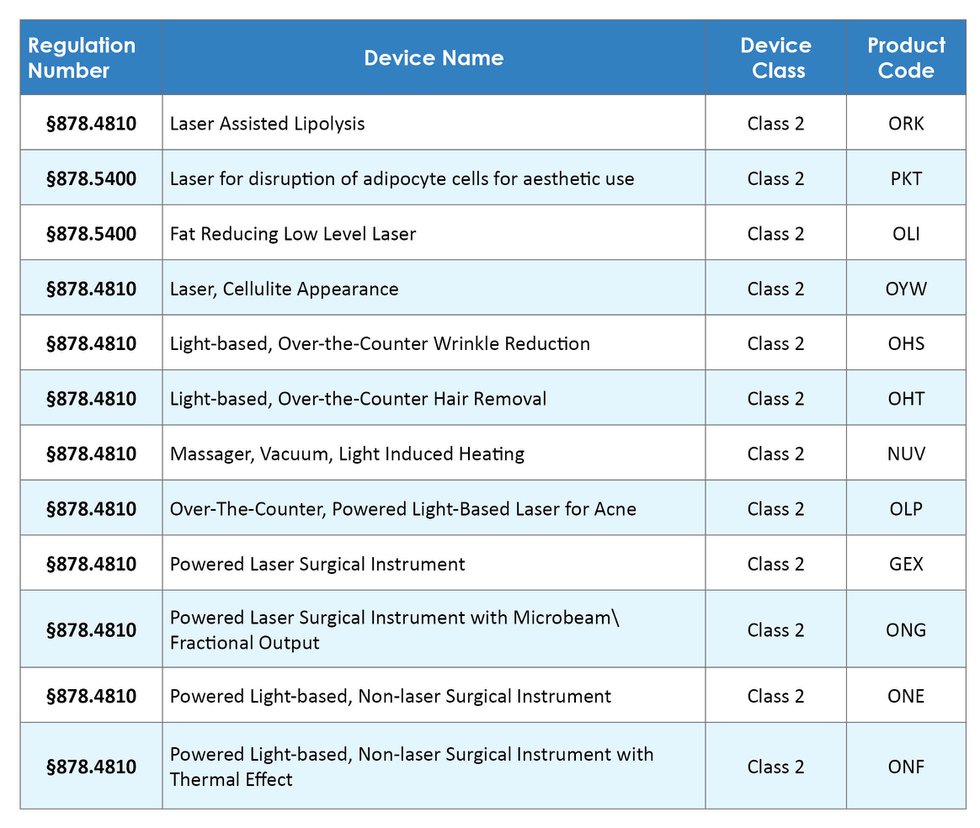
Amazon.com: Pistol Blue Green Laser Light Combo, Multifunction Tactical Flashlight Laser Combo with Strobe Mode, Built-in USB Rechargeable Battery 500 Lumens Flashlight Blue Green Laser Sight for Handguns : Sports & Outdoors
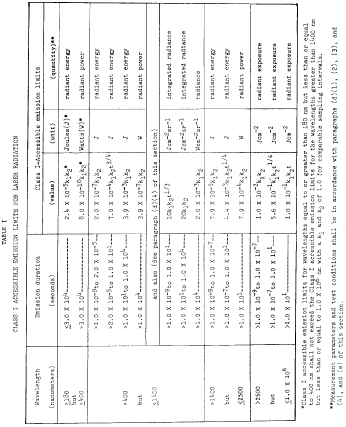
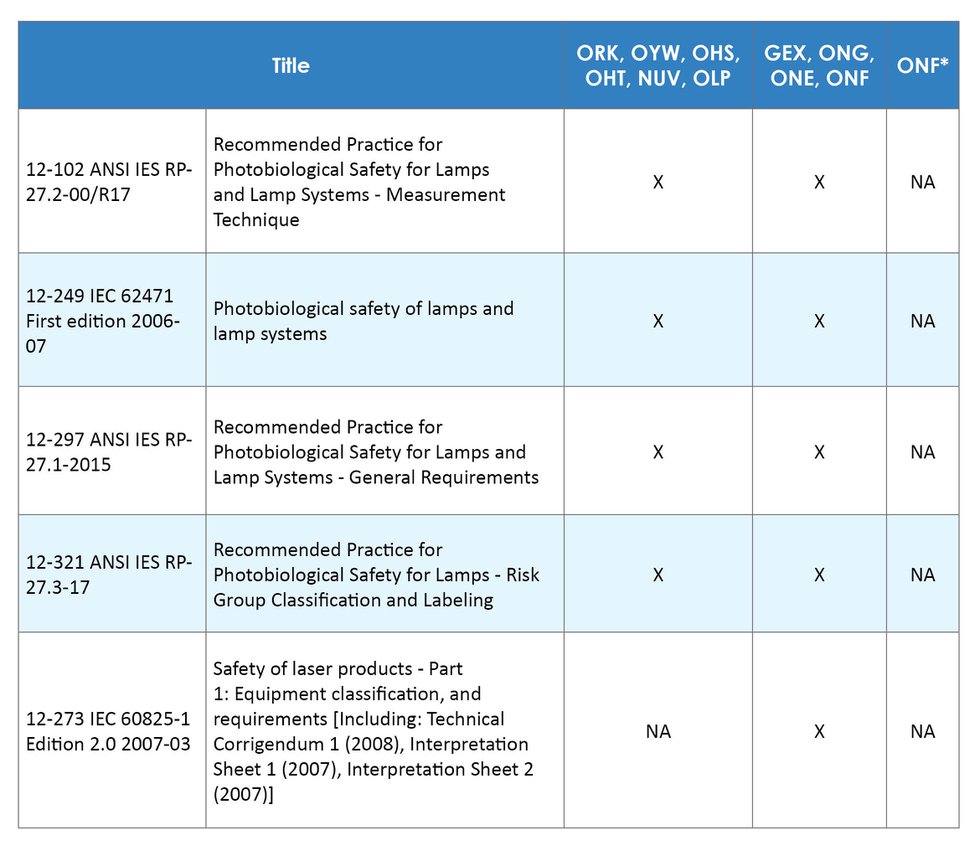
Laser Products - Conformance with IEC 60825-1 Ed. 3 and IEC 60601-2-22 Ed. 3.1 (Laser Notice No. 56) - Guidance for Industry and